Click here for a PDF version of this Calf Note
Introduction
Waste milk – milk collected from cows just after calving and from cows recently treated with antibiotics (during a withdrawal period, when milk may contain excess amounts of antibiotics) – is commonly used as a feed source for young calves.
According to the USDA NAHMS Dairy 2014 study, more than half of all dairy operations and nearly 70% of all heifer calves are fed whole milk (either waste milk or whole milk) prior to weaning for at least some portion of their nutrition. The USDA did not separate saleable from unsaleable milk, but we may assume that a majority of the milk was probably unsaleable (waste) milk.
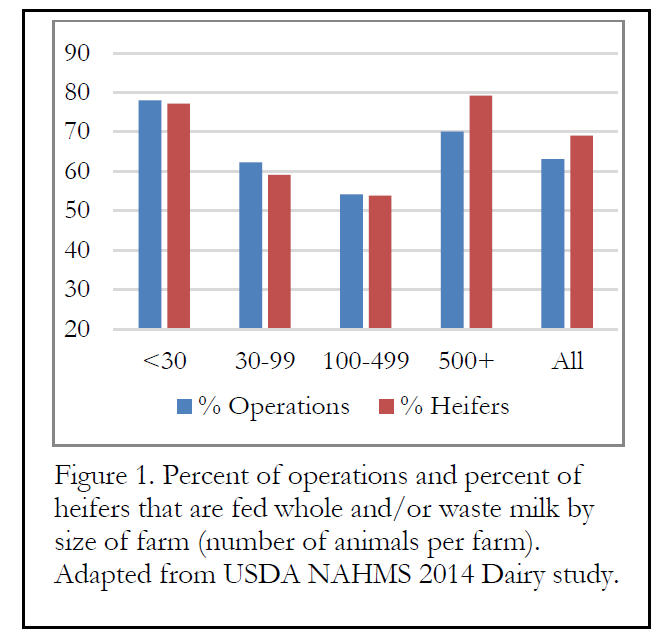
Because waste milk contains at least some antibiotics, the question of development of antibiotic resistance is common. That is, if we feed waste milk, which contains a variable (and usually low) concentration of antibiotics, does this practice increase the chance that calves will development bacteria that are resistant to antibiotics?
We normally assume that a certain concentration of antibiotics is required to induce antibiotic resistance in animals. If the concentration of a certain antibiotic in a sample of milk is too low, the bacteria in the milk will be unaffected and they will not develop resistance to that antibiotic. On the other hand, if the concentration of antibiotic is above the minimal inhibitory concentration (MIC), then growth will be impaired and the bacteria (we assume in the intestine) may develop resistance. On the other hand, Gullberg et al. (2011) reported that drug concentrations up to several hundred-fold below the MIC of susceptible organisms could increase bacterial resistance, even when present at very low concentrations. Therefore, it seems possible that concentrations of antibiotics at concentrations found in waste milk might influence the antibiotic resistance of bacteria in otherwise healthy calves.
Finally, it has been shown that antibiotic resistance genes may be transferred in bacteria in parts of the body other than the intestine. This could have a lot of significance if resistance is found in lungs, where the risk of infection is great, particularly when ventilation is not optimal.
The Research
Researchers at the University of Barcelona (Maynou et al., 2017) used calves on eight dairy farms in the study. Calves on four farms were fed waste milk (WM) and calves on the other four farms were fed milk replacer (MR). The total amount of WM or MR varied by normal management on the farm, although most fed 2 L/feeding in two feedings daily. Calf housing also varied somewhat by farm, though most calves were housed individually for a period of time prior to moving into groups before weaning. The antibiotics typically used (and those tested as part of the study) included amoxicillin, ceftiofur, enrofloxacin, erythromycin, colistin, doxycycline, florfenicol, imipinem, and streptomycin.
Fecal and nasal swabs were collected from about 20 calves on each farm. Calves were about 42 days of age at the time of sampling. Any calf that was treated prior to 42 days of age with antibiotics for diarrhea or respiratory infections was excluded from the study to eliminate the risk of confounding the study. The swabs were analyzed for the presence of antibiotic resistant bacteria.
The Results
The types and concentration of antibiotics in WM used in each of the farms was not monitored during the study; therefore, it is not possible to determine unequivocally the direct concentrations required for each antibiotic in WM.
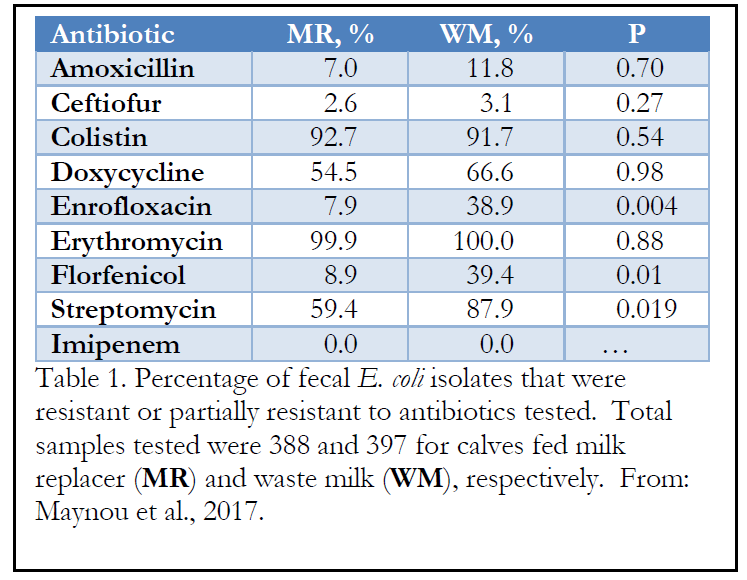
The antibiotics used by the farms to treat or prevent diseases were included as a block in the statistical model. However, this factor did not have any effect on the proportion of resistant E. coli per calf.
Feeding WM increased the proportion of isolates resistant to enrofloxacin, florfenicol and streptomycin (Table 1). In addition, feeding WM increased the percentage of fecal E. coli isolates that were multi-drug resistant. Most of the study farms used enrofloxacin to treat calves for diarrhea and respiratory disease and streptomycin to treat cows with mastitis. On the other hand, only 1 farm used florfenicol and the statistical increase in resistant isolates was not expected. The authors hypothesized that “the use of a particular antimicrobial could select for resistance to other antimicrobials within a bacterial population.” Transmission of resistance genes from one bacterial species to another has been documented.
Most farms used a type of beta-lactam antibiotic, including amoxicillin and ceftiofur to treat mastitis in cows and treat pneumonia and diarrhea in calves. Thus, the authors expected high levels of resistance in fecal samples collected. However, there was not high levels of resistance in fecal E. coli and no effect of feeding WM. At least for this class of antibiotics, other effectors of resistance appear to be important.
Conversely, resistance to doxycycline and erythromycin were quite high in fecal E. coli isolates even though doxycycline was not used on any farm and erythromycin was used on only 1 farm. Again, the authors hypothesized that bacteria-to-bacteria transmission of resistance genes may have occurred via animal-to-animal contact or consumption of contaminated feed or water. Animals from other farms or feed containing resistant bacteria could theoretically contribute to increased resistance in the study.
In addition to evaluating prevalence of antibiotic resistant bacteria in the intestine, the authors collected samples from the respiratory tract and evaluated the prevalence of resistant Pasteurella multocida in both groups. However, only about 36.5% of the nasal samples collected contained P. multocida. Therefore, the estimation of antimicrobial resistance was more difficult to determine. Generally, the contribution of feeding WM to antibiotic resistance of P. multocida was minimal. Only resistance to colistin was increased in P. multocida from nasal secretions when WM was fed. Interestingly, colistin was used on only one farm to treat sick calves (respiratory and digestive problems). Different types of bacterial mutations and horizontal (bacteria-to-bacteria) transmission of resistance genes have been reported in the literature and were hypothesized as a reason for the degree of resistance in P. multocida in this study.
Summary
Feeding waste milk, a common practice in the dairy industry, appears to increase the antibiotic resistance in fecal bacteria of calves (measured by E. coli in this study), and to a lesser extent, respiratory bacteria (measured by P. multocida). Also, the high degree of resistance of both bacteria to antibiotics that were rarely used on never used on the dairies in the study suggest that horizontal transmission of genetic resistance from other bacteria in the environment or other animals may contribute to antibiotic resistance in young calves.
References
Gullberg, E., S. Cao, O. G. Berg, C. Ilbäck, L. Sandegren, D. Hughes, and D. I. Andersson. 2011. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 7:e1002158.
Maynou, G., A. Bach, and M. Terré. 2017. Feeding of waste milk to Holstein calves affects antimicrobial resistance of Escherichia coli and Pasteurella multocida isolated from fecal and nasal swabs. J. Dairy Sci. 100:2682–2694.