Introduction
Coccidiosis, caused by protozoa of the genus Eimeria, results in health and economic problems to several classes of livestock. The disease reduces feed consumption, body weight, and feed efficiency and may cause mortality of 24% in some cases (Fitzgerald, 1975). An estimated 77 million young cattle in the United States could be infected by coccidia during the first year of their life. Of these, 4 million will be treated for coccidiosis, and 80,000 cattle could die from the disease (Fitzgerald, 1975). Annual economic losses due to coccidiosis have been estimated at $62 million (Williams, 1984).
Coccidiosis commonly affects young cattle up to 2 years of age. Animals housed in proximity are more likely to contract the disease. Therefore, feedlot and dairy cattle are most susceptible. Coccidiosis in feedlot cattle is associated with stress caused by shipping, changes in ration and in weather, and overcrowding (Ernst and Benz, 1986). Stress caused by weaning makes dairy calves very susceptible to coccidiosis.
Etiology
All domestic animal species are susceptible to coccidial infections. Although coccidia are host- specific, each host may be infected with several species of coccidia at the same time. At least 13 different coccidial species are known to infect cattle in the United States (Ernst and Benz, 1986), but not all are pathogenic. The two most pathogenic species are Eimeria bovis and Eimeria zuernii (Ernst and Benz, 1986). Incubation periods for E. zuernii and E. bovis are usually 15 to 20 days (Georgi, 1985). Immunity to coccidiosis persists only 3 to 4 months, and reinfection may occur in the absence of continuous challenge (Fitzgerald, 1975).
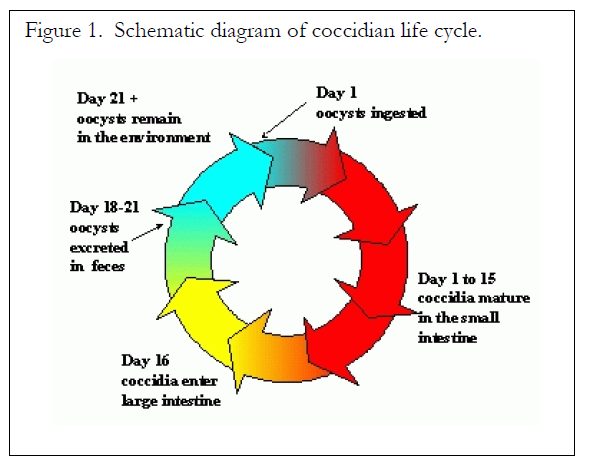
Coccidia usually infect epithelial cells of the gut mucosa during the developmental stage (Ernst and Benz, 1986), but there are exceptions. For example, large, first-generation schizonts of E. bovis and E. zuernii can be found in endothelial cells of the central lacteals in the small intestine (Hammond et al., 1946), and in connective tissue cells of the lamina propria (Stockdale, 1976), respectively.
Coccidiosis is transmitted by ingestion of sporulated oocysts. Infection is acquired from contaminated feed, water, and soiled pastures, or by licking a contaminated hair coat (Georgi, 1985). Signs of the disease include anorexia, loss of weight, and hemorrhagic and mucoid diarrhea (Georgi, 1985). In severe cases, feces are liquid, bloody and may contain strands of intestinal mucosa (Ernst and Benz, 1986). Animals may become emaciated, dehydrated, weak, and listless. Rectal prolapse may result from straining without defecation (Ernst and Benz, 1986).
The clinical course of coccidiosis ranges from 4 to 14 days, and the mortality rate may be as high as 24% in severe outbreaks (Fitzgerald, 1975). Death is primarily a result of diarrhea, which causes a loss of electrolytes and dehydration; however, hemorrhaging or secondary complications such as opportunistic infections may contribute to mortality. Animals that recover from severe infections may suffer permanent production losses (Ernst and Benz, 1986).
Clinical and subclinical coccidiosis cause economic and health problems for cattle producers. Both forms result in a decline of herd condition and, if left untreated, mortality can occur. Clinical coccidiosis pertains to responses elicited by the course of the disease, i.e. the signs. It has been estimated that 5% of infected animals show clinical signs of coccidiosis (Muirhead, 1989). Subclinical coccidiosis, in contrast, refers to a period before appearance of typical signs of the disease, or infected animals that do not show signs of a clinical infection. The remaining 95% suffer from subclinical coccidiosis, which can result in increased economic loss (Muirhead, 1989). Since subclinical coccidial infections do not exhibit signs of the disease, animals could be infected without cattle producer’s knowledge. Losses due to clinical and subclinical coccidiosis result from a decrease in absorption of nutrients due to damage to the intestinal lining (Muirhead, 1989).
Mortality from coccidiosis is usually associated with severe diarrhea, which causes loss of electrolytes and dehydration. In one study, calves with diarrhea lost 8 and 18 times more sodium and potassium respectively, than normal calves (Blaxter and Wood, 1958). Denatured proteins cause shifts in osmotic pressure, and alter levels of intra- and extra-cellular ions (Fitzgerald, 1967, Roy et al., 1959). Coccidia destroy intestinal cells, which results in loss of blood and other fluids into the small intestine (Blaxter and Wood, 1958). Blood and other fluid then pass in the feces, which are usually watery. When schizonts are mature, intestinal cells are sloughed from membranes and either leave scattered epithelial cells to cover the lamina propria or expose lamina propria with engorged capillaries (Blaxter and Wood, 1958). If these exposed capillaries are severely damaged, blood and plasma may be lost.
Cattle producers and veterinarians have problems diagnosing coccidiosis because clinical signs are associated with the late portion of the early sexual phase (Muirhead, 1989). Passage of oocysts follows signs of coccidiosis, therefore, if there are large numbers of oocysts in the feces, coccidia probably have already completed their life cycle (Ernst and Benz, 1986). If treatment is given at this time, and secondary bacterial infections are controlled, animals will probably recover (Georgi, 1985).
Treatment
Although coccidiosis is considered a disease of young animals, older animals are frequently infected with Eimeria. The severity of clinical coccidiosis depends on the number of sporulated oocysts ingested and the general health of the infected host (Ernst and Benz, 1986). An objective of control could thus be reducing the number of oocysts available for ingestion. However, no minimum infective dose for coccidia has been established (Muirhead, 1989). Proper sanitation and good animal husbandry practices are important in preventing coccidiosis. Water and feed troughs should be constructed and located to reduce fecal contamination. Newborn dairy calves should be provided with clean, dry quarters when re moved from the dam (Ernst and Benz, 1986).
A major difficulty in treating clinical coccidiosis is that signs of the disease do not appear until the life cycle is almost complete. By this time, the gut may be severely damaged. Most anticoccidial drugs are only effective during early stages of a coccidian life cycle. Thus, the difficulty in treating coccidiosis is that by the time signs appear, parasites have already passed through the stage in which anticoccidial drugs are most effective. Infected animals often recover without treatment due to acquired resistance to the disease (Blaxter and Wood, 1958). However, treatment with anticoccidial drugs should be administered at the earliest clinical signs because it may reduce severity of the disease and decrease mortality. Antibiotics may be administered to reduce secondary infections. Electrolyte solutions and fluids should be administered to control dehydration. During treatment, animals should be isolated in a clean environment to prevent further contamination.
Treatments for coccidiosis include sulfonamides in the drinking water and amprolium in the feed. Polyether antibiotics, such as lasalocid and monensin, originally developed as coccidiostats for poultry, have been effective in preventing coccidiosis in cattle.
Decoquinate aids in controlling coccidiosis caused by E. bovis and E. zuernii in calves and older cattle. Decoquinate fed at 0.5 mg/kg body weight for at least 28 days during periods of exposure to sporulated oocysts aids in controlling the disease (Miner and Jensen, 1976). For decoquinate to be effective for cattle, it must be fed to provide 22.7 mg/100 lb. of body weight/head/day (Muirhead, 1989). Research with decoquinate at North Carolina State University (Ramsey et al., 1991) shows that the drug can be effective in increasing feed intake and gain when animals are placed in an environment where coccidia have been found previously.
Monensin fed at 1 mg/kg body weight/day throughout the incubation period of an experimental infection of E. bovis prevented development of clinical signs of coccidiosis (Fitzgerald and Mansfield, 1973). However, monensin did not have total coccidiostatic effects. Monensin fed at 16.5 or 33 g/metric ton of feed for 31 days prevented development of clinical signs of coccidiosis (McDougald, 1978).
Studies have evaluated lasalocid as a coccidiostat using different concentrations in the feed (Conlogue et al., 1984). However, the recommended level of lasalocid to control coccidiosis is 1 mg/kg body weight (Muirhead, 1989), which reduces fecal oocyst shedding in calves with natural exposure to coccidiosis (Hoblet et al., 1989). Lasalocid at 33 to 44 mg/kg of the diet was highly effective against naturally occurring coccidiosis in feedlot cattle (Horton and Brandt, 1981).
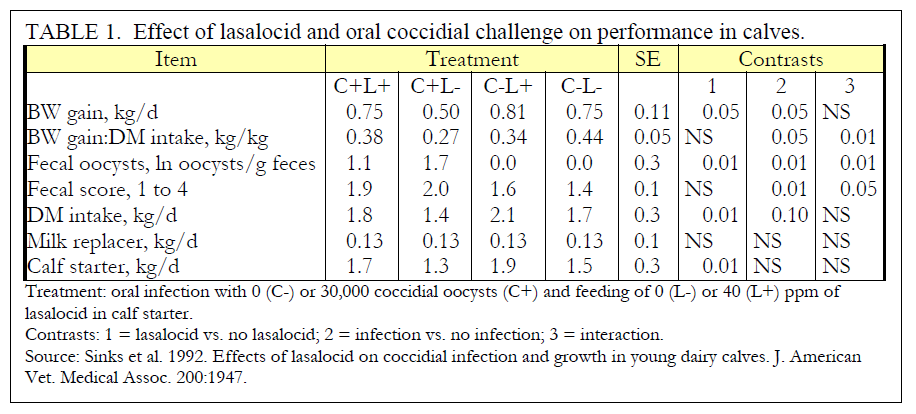
Because ionophores have both anti-coccidial and growth promoting properties, it may be important to determine if responses in body weight and gain are a response to coccidial control or altered ruminal fermentation. We conducted a study (Sinks et al., 1992) to determine the nature of response to lasalocid in young calves. Sixteen Holstein bull calves were assigned randomly at birth to a 2 x 2 factorial arrangement of calf starter containing 0 (L-) or 40 ppm (L+) lasalocid, and single oral infection with 0 (C-) or 30,000 (C+) sporulated oocysts (E. bovis) administered 28 days after initiation of the study. Sporulated oocysts were administered orally in a single gelatin capsule.
Gain-to-feed ratio was reduced when calves were infected with C+, but a highly significant interaction (P < 0.01) indicated that calves on C+L- treatment had markedly reduced feed efficiency. Gain-to-feed ratio was most affected during weeks 7 and 8, in calves inoculated with coccidia. Average gain-to-feed ratios were 0.29, -0.08, 0.41, and 0.41 for C+L+, C+L-, C-L+, and C-L- treatments, respectively, during weeks 7 and 8.
Logarithms of coccidial numbers (Table 1) showed a marked increase in calves infected with C+. However, in calves fed L+, log of fecal oocysts after inoculation was reduced (P < 0.01) from 1.71 to 1.14. Oocysts (all E. bovis) began appearing in feces of C+L- and C+L+ calves approximately 21 days after infection. Calves receiving L+ shed fewer oocysts than untreated calves (P < 0.01). Approximately 4 weeks after infection, numbers of oocysts shed by calves infected with C+ declined through the end of the study, suggesting that calves developed resistance to the coccidia, or that no reinfection by coccidial oocysts occurred. Lasalocid effectively reduced the number of oocysts shed by calves on treatment C+L+, although coccidiostatic effect was not complete.
Diarrhea was observed in calves 3 weeks after coccidial infection. Calves on C+L- treatment developed moderate to severe diarrhea (fecal score > 2) during the shedding of oocysts, and one of these developed bloody diarrhea. Calves on C+L+ treatment developed moderate diarrhea (fecal score > 2) during the shedding of oocysts (Table 1). One calf on C-L+ treatment had diarrhea during the study. Feces from this calf did not contain oocysts or blood.
Results of this study indicate clearly effects of coccidial challenge with a moderate dose of E. bovis on growth, intake, and some metabolic parameters of calves after four weeks of age. Effects of challenge were apparent in calves on C+L- treatment approximately 3 weeks after dosing. Body weights declined during weeks 6-8; thereafter calves may have developed natural resistance to coccidia. Body weight gain was reduced only when fecal oocysts exceeded approximately 1,000/g feces, during weeks 7 and 8. At no time did fecal oocysts reach this level in calves on C+L+ treatment and gain was unaffected. Calves not infected with coccidia never discharged oocysts, indicating no cross-contamination between treatment groups. It should be noted that calves were housed in a barn previously uninhabited by other cattle, therefore, the absence of oocysts in feces of non-inoculated calves was expected. Feed efficiency was also influenced by coccidial challenge. Reduction in feed efficiency of nearly 30% was probably due to a loss of nutrient absorption in the small intestine. Coccidia destroy the lining of the small intestine, causing nutrients to be incompletely absorbed (Ernst and Benz, 1986). Anorectic effects of coccidiosis, (Williams, 1984) coupled with depressed intestinal absorption would markedly reduce feed efficiency in calves on C+L- treatment.
Although intake of lasalocid was generally lower than 200 mg/head/d fed for maximal growth responses, it appeared that lasalocid did affect the environment of the developing rumen, and subsequent growth. Rates of gain were higher than other reports in our laboratory (Quigley et al., 1991) due to the influence of lasalocid. Additionally, intake of feed was greater in calves fed lasalocid during the last six weeks of the study, suggesting that as ruminal function was established, lasalocid modified the ruminal environment. Anderson et al. (1988) also reported increased feed intake in calves fed calf starters containing 44 ppm lasalocid after six weeks of age, as well as increased rates of ruminal fermentation and microbial numbers. Apparently, lasalocid altered intake and growth by increasing ruminal fermentation as well as control of coccidia.
References
Anderson K. L., T. G. Nagaraja, J. L. Morrill, P. G. Reddy, T. B. Avery, and N. V. Anderson. 1988. Performance and ruminal changes of early-weaned calves fed lasalocid. J. Anim. Sci. 66:806.
Blaxter, K. L., and W. A. Wood. 1958. Some observations on the biochemical events associated with diarrhea in calves. Vet Rec. 65:889.
Conlogue, G., W. J. Foreyt, and R. B. Wescott. 1984. Bovine coccidiosis protective effects of low-level infection and coccidiostat treatments in calves. Am. J. Vet. Res. 45:863.
Ernst J.V., and G. W. Benz. 1986. Intestinal coccidiosis in cattle. The veterinary clinics of North America/parasites: epidemiology and control. W.B. Saunders Company, Philadelphia, PA.
Fitzgerald, P. R. 1967. Effect of bovine coccidiosis on blood serum sodium and potassium levels of calves. Am. J. Vet. Res. 28:667.
Fitzgerald, P. R. 1975. The significance of bovine coccidiosis as a disease in the United States. Bovine Pract. 10:28.
Fitzgerald, P. R., and M. E. Mansfield. 1973. Efficacy of monensin against bovine coccidiosis in young Holstein-Friesian calves. J. Protozool. 20:121.
Georgi, J. R. 1985. Parasitology for veterinarians. Fourth ed. W. B. Saunders Co., Phila. PA.
Hammond, M., G. W. Bowman, L. R. Davies, and B. T. Simms. 1946. The endogenous phase of the life cycle of Eimeria bovis. J. Parasit. 32:409.
Hoblet, K. H., T. P. Charles, and R. R. Howard. 1989. Evaluation of lasalocid and decoquinate against coccidiosis resulting from natural exposure in weaned dairy calves. Am. J. Vet. Res. 50:1060.
Horton, G. J., and W. E. Brandt. 1981. Lasalocid or monensin for finishing steers fed a high- silage diet. J. Anim. Sci. 53(Suppl. 1):406 (Abstr.).
McDougald, L. R. 1978. Monensin for the prevention of coccidiosis in calves. Am. J. Vet. Res. 39:1748.
Miner, M. L., and J. B. Jensen. 1976. Decoquinate in the control of experimentally induced coccidiosis of calves. Am. J. Vet. Res. 37:1043.
Muirhead, S. 1989. Coccidiosis infections often go undetected in beef, dairy cattle. Feedstuffs. 15:87.
Quigley, J. D., III, L. A. Caldwell, G. D. Sinks, and R. N. Heitmann. 1991. Blood glucose, nonesterified fatty acids and ketones in response to weaning and feed intake in young calves. J. Dairy Sci. 74:250.
Radostits, O. M., and P. H. Stockdale. 1980. A brief review of bovine coccidiosis in western Canada. Can. Vet. J. 21:227.
Ramsey, H. A., L. W. Whitlow, B. T. McDaniel, and G. A. Ducharme. 1991. Protective effect of decoquinate for preruminant Holstein calves in relation to serum Ig. J. Dairy Sci. 74(Suppl. 1):273.
Roy, J. B., K. W. Hawkins, H. Gillian, J. M. Lang, and P. L. Ingraham. 1959. The effect of white scours on the sodium and potassium concentration in the serum of newborn calves. Br. J. Nutr. 13:219.
Sinks, G. D., J. D. Quigley, III, and C. R. Reinemeyer. 1992. Effects of lasalocid on coccidial infection and growth in young dairy calves. JAVMA. 200:1947.
Stockdale, P. G. 1976. Schizogony and gametogony of Eimeria zuernii (Rivolta, 1878) Martin, 1909. Vet. Parasit. 1:367.
Williams L. 1984. Dealing with the frustration of the war on coccidiosis. DVM. 15:38.