Click here for a PDF version of this Calf Note
Introduction
Several recent Calf Notes have looked into the causes of sub-acute rumen acidosis (SARA) in cows and calves (#170, #172, and #173). This note is intended to evaluate effects of SARA on the health of cows and calves.
How does SARA affect health?
In a terrific recent review of the effects of SARA on cow health, Plaizier et al. (2012) documented in great detail effects of grain-based challenges on cow health. When cows (and, by extension, calves) eat lots of grain in a short period of time (or when they are challenged with large amounts of grain), there is typically a reduction in the pH of the rumen, which can result in sub-acute rumen acidosis, or SARA. Research suggests that many, if not most, calves experience SARA at some point during the rumen development period. In most research, SARA is defined as a rumen pH <5.8. When pH falls to this level, fiber digestion is depressed and the cow (calf) may experience various metabolic disturbances such as an on-off-on cycle of feed intake, loose manure, and depressed performance, among other problems.
A key component of SARA on animal health is the production of lipopolysaccharide (LPS) that results from the death of gram-negative bacteria. Also called endotoxin, LPS is a signal to the body that a bacterial invasion has occurred, and the body responds by mobilizing the immune response to fight off this new infection. As a result, there are several clear signs caused by LPS – fever, production of pro-inflammatory cytokines such at TNF-α, anorexia, and, in severe cases, toxic shock and death.
Normally, LPS is produced in the rumen as a result of normal rumen bacterial cell growth and death. However, concentrations are generally low, and intestinal systems detoxify LPS that leaves the rumen. For example, abomasal proteases, lysozyme and hydrochloric acid, contribute by killing or inhibiting bacteria. Low abomasal pH can also deactivate LPS (Ribeiro et al., 2010). Bertok (1998) also reported that bile acids cause degradation of LPS in the small intestine.
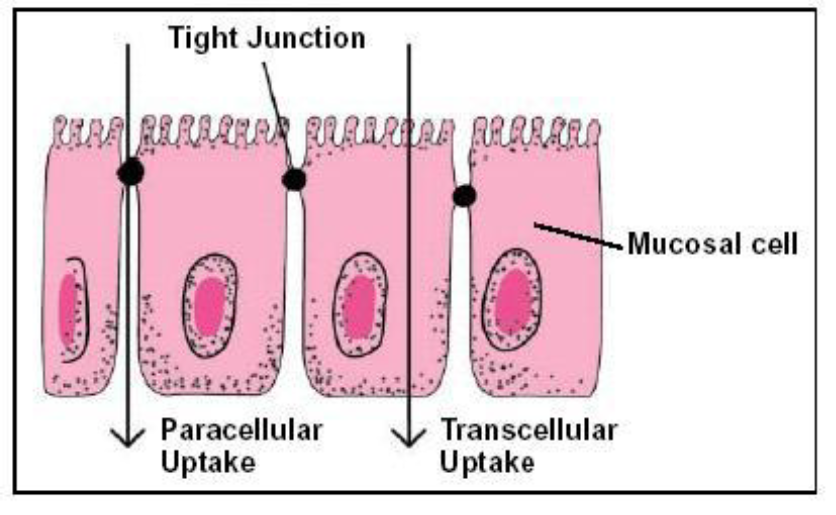
In the case of a grain challenge (intake of a large “slug” of grains containing large amounts of starch), the amount of LPS in rumen fluid has been shown by several authors to increase. Under certain conditions, the amount of LPS taken up into the blood can increase. One possible reason for this increase is the effects of stress on tight junction proteins. Tight junctions are proteins between intestinal epithelial cells (see Figure 1) and generally limit the movement of molecules between cells (i.e., paracellular uptake). Thus, most nutrients and other molecules must be absorbed by the cells (transcellular uptake). However, under certain conditions, tight junction proteins lose their ability to control movement of substrates from the lumen of the intestine, and the amount of paracellular uptake increases dramatically. This is often referred to as “leaky gut” and it is caused by a number of factors, including antigens, microorganisms, cytokines, cold/heat, changes in diet (e.g., weaning) and other factors. The increased permeability allows increased uptake of LPS and causes a corresponding immune response.
Effects of SARA induced immune response
Data suggest that SARA can cause LPS to move from the intestine into the peripheral circulation. When LPS reaches the blood, there is a systemic response, which causes production of acute phase proteins, changes in utilization of nutrients and reduced efficiency of production. In lactating cows, increased plasma acute phase proteins are associated with reduced milk fat, 3.5% fat corrected milk and efficiency of production (Dong et al., 2011).
Where does the LPS come from?
Conventional wisdom suggested that LPS produced by death of rumen bacteria was the primary cause of LPS that eventually reached the blood stream and caused reduced performance. However, Plaizier et al. argue that another source of LPS in the blood comes from high acidity of digesta in the large intestine. They argue that the level of starch reaching the small and large intestine is an important consideration. That is, starch that is not fermented in the rumen (due to large starch intake coupled with high rate of passage) and not digested by the small intestine may reach the large intestine, where it can be fermented by large intestinal bacteria, producing large amounts of fermentation acids. These acids and bacterial death occurring in the large intestine may also impair tight junctions and result in LPS crossing into the circulation.
What effects on calves?
Though there appears to be clear evidence that SARA exists in cows and can have significant health effects, there are few data to suggest similar effects on calves. Clearly, however, there are suggestions that similar physiological mechanisms could play a role in calves.
Immediately after weaning, it’s common for starter intake to increase several fold in a matter of days, as calves attempt to replace the energy previously provided by milk. This has the effect of doubling or tripling the load of carbohydrate presented to the rumen and rumen bacteria to process. Since bacteria in the rumen are adept even early in life to ferment carbohydrates such as starch and cellulose in the rumen, this would result in a rapid increase in VFA production, microbial growth, and potentially in LPS production in the rumen. The combined effect of low pH, high acidity, and LPS production can result in translocation of bacteria across the rumen wall, development of rumen parakeratosis and liver abscesses. None of these effects are beneficial to the calf.
When calf starter diets contain a lot of starch, a scenario (as proposed by Plaizier et al.) might proceed as follows – rapid increase in starter intake with weaning. Since the starter contains a large amount of starch, there may be increase escape of starch from the rumen. Starch digestion in the rumen, small intestine and large intestine varies widely among animals and diets (Huntington et al., 2006). Should more starch reach the large intestine, it could initiate production of LPS and subsequent immune response by breakdown of gut integrity and migration of LPS into the circulation.
At the time of this writing, this hypothesis is conjecture. There are no data to suggest that ruminal or intestinal LPS increases in just weaned calves, nor that there is an immune response to such an increase.
However, we know that weaning stress can result in immune suppression (e.g., Hulbert et al., 2011; Kim et al., 2011). Interestingly, Kim et al. (2011) reported increases in acute phase proteins and concentrations of tumor necrosis factor alpha in just weaned calves, which is consistent with the hypotheses posed here. Further research is needed to determine if our methods of weaning and composition of starter fed around weaning might affect calf immunity and predisposition to disease.
References
Bertok, L., 1998. Effect of bile acids on endotoxin in vitro and in vivo (physico-chemical defense): bile deficiency and endotoxin translocation. Ann. N.Y. Acad. Sci. 851:408–410.
Dong, G., S. Liu, Y. Wu, C. Lei, J. Zhou, and S. Zhang. 2011. Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: impacts on immunity and metabolism. Acta Vet. Scand. 53:48-54.
Hulbert, L.E., C. L. Cobb, J. A. Carroll, and M. A. Ballou. 2011. The effects of early weaning on innate immune responses of Holstein calves. J. Dairy Sci. 94:2545-2556.
Huntington, G. B., D. L. Harmon, and C. J. Richards. 2006. Sites, rates, and limits of starch digestion and glucose metabolism in growing cattle. J. Anim. Sci. 84(E. Suppl.):E14-E24.
Kim, M.H., J. Y. Yang, S. D. Upadhaya, H. J. Lee, C. H. Yun, and J. K. Ha. 2011. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J. Vet. Sci. 12:151-157.
Plaizier, J. C., E. Khafipour, S. Li, G.N. Gozho, D.O. Krause. 2012. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 172:9–21.
Ribeiro, M.M., Xu, X., Klein, D., Kenyon, N.S., Ricordi, C., Felipe, M.S., Pastori, R.L., 2010. Endotoxin deactivation by transient acidification. Cell Transplant. 19:1047–1054.